What is the HuPEX® comprehensive protein array analysis service?
Our HuPEX® comprehensive protein array analysis service can analyze antibody reactivity to approximately 13,500 types of human proteins at once.
The protein arrays technologies used in this service were developed by the National Institute of Advanced Industrial Science and Technology (AIST). We were independent from AIST, and have an exclusive licensing agreement with AIST.
This protein array has been used in numerous collaborative research projects since the days of AIST, and has produced numerous research results, including the comprehensive analysis of autoantibodies in human blood, evaluation of the reactivity of purified antibodies, discovery of new biomarkers, and antigen identification.
Proteobridge Co., Ltd. has further developed these technologies and launched HuPEX array as a contract analysis service in September 2023, which is now being used by many medical research institutions and pharmaceutical companies.
Representative diseases in which autoantibodies are disease markers/ causes
The HuPEX🄬 comprehensive protein arrays keep non-dried state and retain the structures of 13,500 antigen proteins included.Therefore, antigen-antibody reactions can be easily reproduced on the protein array substrate. HuPEX🄬 comprehensive protein arrays are better for identifying autoantibodies.
Autoantibody-related diseases are representative autoimmune diseases.
Autoantibodies are known to be biomarkers for various diseases and to cause the onset of diseases. The research into the relationship between diseases and autoantibodies is on the rise around the world.

Autoimmune disorders
Autoimmune diseases are a general term for diseases that occur when the immune system, which normally attacks foreign substances that enter the body from outside, judges our body’s protein as a foreign substance and attacks our body as a result of an abnormal autoimmune reaction.
These diseases are rare, and have not been elucidated. Because of this, the government often designates them as incurable. For many of these diseases, the presence or absence of autoantibodies is an item in the diagnostic criteria.
Connective tissue disease
Connective tissue disease, a category of autoimmune disease, is a general term for a number of diseases.This diseases patients have cells (autoreactive lymphocytes) and proteins (autoantibodies) that attack the body themselves, which seems cause inflammation in the skin, muscles, joints, internal organs, blood vessels, etc.
The representative connective tissue disease include rheumatoid arthritis, systemic lupus erythematosus (SLE), Sjögren’s syndrome, polymyositis/dermatomyositis, and systemic sclerosis.
HuPEX® comprehensive protein array analysis service details
Service Details
| item | Contents |
|---|---|
| Service Name | HuPEX🄬 Comprehensive protein array analysis service |
| Overview of the inspection | HuPEX🄬 comprehensive protein array analysis service is a contract service specializing in comprehensive antibody profiling of serum, plasma, and purified antibodies against human proteins. |
| Applications | Biomarker discovery, antigen identification, disease monitoring, comparison before and after treatment, specificity verification of drug candidate antibodies, etc. |
| Number of antigens detected (based on gene name) | 13,351 types (as of August 22, 2023) |
| Sample type and volume | 300 μL of serum or plasma *Please inquire about purified antibodies |
| Conditions for submitting samples | After blood collection, please separate the serum and provide us with the frozen sample. The antibody will be determined after consultation. |
| Minimum order quantity | 2 samples |
| Inspection method | Indirect immunofluorescence |
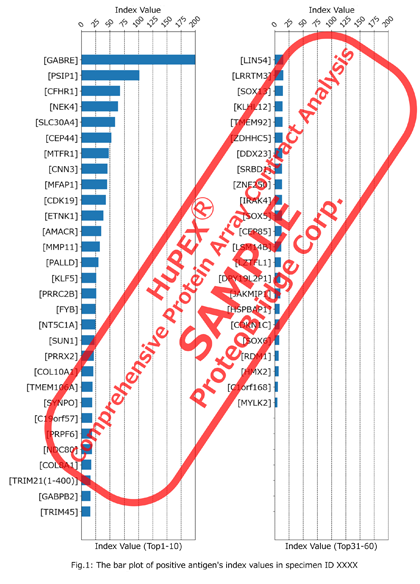
| Report Format | Result report (PDF format) Quantitative data for each protein (Excel format) *Quantitative data will be reported as index values. * Comparison between arrays is possible. |
| Required time | In principle, within two months from receipt of the sample |
| Inspection fee | Separate quote required |
| How to request an inspection | Please refer to the inspection information and inspection request form on our website. |
| note | We provide autoantibody data (Index values) from healthy adults for free cost. This service is provided for research purposes only and is not to be used for diagnostic purposes. |
File download:*Preparing
Results report:*Preparing
HuPEX® Option Analysis Service
Analysis Graph
*Photo is for graph purposes only.
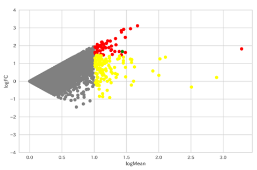
・MA plot
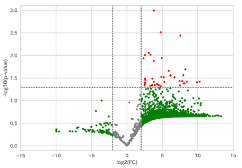
・Volcano plot
・Correlation analysis
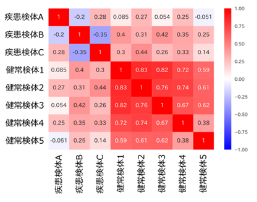
・Heatmaps and Clustering
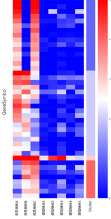
*Photo is for graph purposes only.
The following services will be provided in Excel format. :*Preparing
| Analysis items | overview | price |
|---|---|---|
| MA plot* | We graph the relationship between the fold change and the autoantibody response between the two groups. This allows us to select autoantibody candidates for which significant differences are likely to be observed. (The number of samples per group does not matter.) | ¥20,000-/1pattern*** |
| Volcano plot* | A comparative analysis between the two groups is carried out, the significant difference P value and the ratio of antibody reactions are shown, and autoantibodies with significant differences are extracted. (At least three samples are required per group.) | ¥30,000-/ 1pattern*** |
| Correlation analysis | A graph is created to show how similar each autoantibody is between the two groups. | ¥10,000- |
| Heatmaps and Clustering | We classify the results by autoantibody reaction and create a graph in which the amount of autoantibody reaction is represented by color. | ¥30,000- |
| Expression analysis in normal tissues** | We estimate the healthy human tissues and cell types in which a given autoantigen is expressed. | ¥20,000-/ 1pattern*** |
| Expression analysis in cancer cells** | We perform estimation of cultured cancer cells expressing any autoantigens. | ¥20,000-/ 1pattern*** |
| Site of antigen expression within a cell** | We predict the organelle in which any autoantigen is expressed. | ¥20,000-/ 1pattern*** |
* MA plot and Volcano plot are either one or the other. The former is for reference only, as no significance test was performed. The latter analysis involves significance testing.
** This analysis can be performed for candidate or positive autoantibodies with significant differences.
*** For example, if there are groups A, B, and C and the autoantibody reactions of group A vs. group B and group A vs. group C are compared, there will be two patterns. If a brute force comparison of group B vs. group C is added, there will be three patterns.
It is also possible to perform comparative analysis with healthy adult samples held by our company.
Measurement principle
React serum/plasma with the array

Autoantibodies bind antigenic proteins
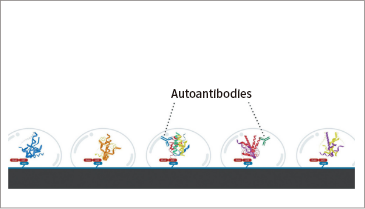
Autoantibodies are detected
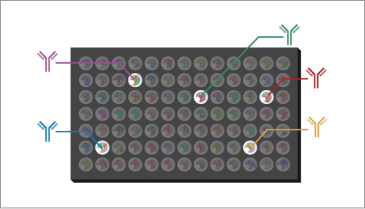
In our comprehensive protein array analysis service, samples (serum, plasma, cerebrospinal fluid, purified antibodies) received from customers are added to the comprehensive protein arrays manufactured by our company and reacted. Measurements are performed by indirect immunofluorescence (IIF), and quantitative data for each antibody is reported.
Measurement procedure
- The sample is added to the protein array and reacts with the antigens (human proteins) present on the array.
- After washing, a fluorescently labeled antibody is added to initiate the secondary reaction.
- The resulting [antigen + antibody + fluorescently labeled antibody complex] is measured using a fluorescent scanner.
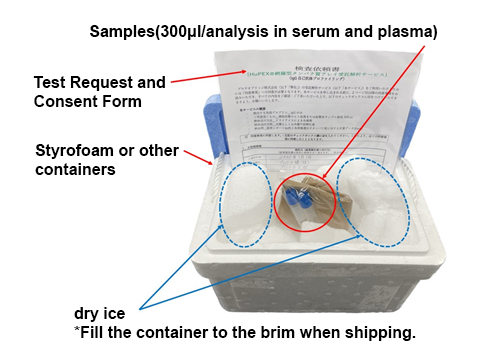
How to order
When you send us your blood sample (serum or plasma), we assay your sample using the HuPEXⓇ comprehensive protein array, analyze and quantify your sample, and create a test report.
Please prepare the sample, test request form, and consent form by referring to the packaging example, and send them to our Contract Inspection Department. We ask that you please bear the cost of shipping the sample.
When sending your sample,please email us with the expected arrival date.
(ProteoBridge Co., Ltd. Contract Testing Division、hupex@proteo-bridge.co.jp)
Sample packaging example
Inspection request form
*Preparing
【Sample delivery address】
2-4-7 Aomi, Koto-ku, Tokyo 135-0064
National Research and Development Agency National Institute of Advanced Industrial Science and Technology, Rinkai Center Annex, Room 5108
Proteobridge Co., Ltd., Contract Testing Division
TEL:03-6457-1661
Examples of HuPEX® Comprehensive Protein Array and Custom Protein Array Applications
Users’ Utilization Results

Case 1: Discovery of a new biomarker, anti-ZSCAN1 autoantibody
Research Methodology
HuPEX® comprehensive protein array analysis
The sample used for array analysis was a mixture of sera from five pediatric patients with ROHHAD syndrome (four females and one male).
As a result, 33 proteins were listed as candidate antigens for autoantibodies.

Custom array creation and autoantibody analysis
A custom protein array was created using 33 types of antigen proteins as candidate markers.
The samples used for array analysis were serum samples from five non-cancer ROHHAD syndrome patients. Autoantibodies reacting with the ZSCAN1 protein were detected in four of the five patients.

Tissue staining with patient serum IgG and anti-ZSCAN1 antibody (commercially available)
Histological staining of mouse extrapituitary organs was performed using patient serum IgG and anti-ZSCAN1 antibody (commercially available).We confirmed that ZSCAN1 protein is expressed in the subfornical organ and that autoantibodies in patient sera react with the same site.ZSCAN1 was identified as an autoantibody target in ROHHAD syndrome.

Development of an ELISA method to detect anti-ZSCAN1 antibodies
We analyzed for positive anti-ZSCAN1 autoantibodies using ELISA within fourteen ROHHAD syndrome non-cancer ROHHAD syndrome patients, 15 normal subjects, and 5 autoimmune diseases patients.
As the result, 12 of 14 patients with ROHHAD syndrome without tumors were confirmed to be positive for anti-ZSCAN1 autoantibodies by ELISA.
Results
◎ These results suggest that anti-ZSCAN1 autoantibodies may be useful as a diagnostic marker for ROHHAD syndrome, regardless of the presence or absence of tumor.
Paper information
Akari Nakamura-Utsunomiya , et al. Anti-ZSCAN1 Autoantibodies Are a Feasible Diagnostic Marker for ROHHAD Syndrome Not Associated with a Tumor
Int J Mol Sci. 2024 Feb 1;25(3):1794. doi: 10.3390/ijms25031794.

The sum of autoantibodies detected by comprehensive protein arrays reflects disease status
Research Methodology
Autoantibody profiling using HuPEX🄬 comprehensive protein arrays
Using the HuPEX🄬 comprehensive protein array loaded with antigen proteins synthesized from 12,946 gene clones, autoantibodies were measured in pooled sera from patients with systemic sclerosis (SSc) (N=32), psoriasis vulgaris (PsO) (N=8), cutaneous arteritis (CA) (N=16), malignant melanoma (N=40), and healthy controls (N=20).

Narrowing down autoantibodies and correlation analysis
Proteome-wide autoantibody profiling was performed in SSc, Pso, and CA, and a total of 565 autoantibodies were detected.We narrowed autoantibodies down to a total of 178 types, including autoantibodies that have been reported as diagnostic or disease activity markers, and autoantibodies against proteins expressed on the cell membrane or outside the cell, and performed a detailed analysis of the clinical findings.

Correlation between clinical characteristics of each disease and autoantibody levels
The sum of autoantibodies (SAL-P-CRP) positively correlated with the inflammatory marker C-reactive protein (CRP) was determined, and the clinical characteristics of each disease were analyzed. In SSc patients, SAL-P-CRP was significantly correlated with interstitial lung disease (ILD) indicators and mRSS, and was shown to be superior to existing markers in predicting ILD.
In Pso patients, SAL-P-CRP was significantly higher in those with psoriatic arthritis (PsA) and correlated with skin symptoms (PASI score). In CA patients, SAL-P-CRP was significantly higher in those with non-responsiveness to treatment.

Clinical significance of comprehensive measurement of autoantibodies in malignant melanoma
We detected 488 autoantibodies in MM patient sera.It was found that autoantibody production was more pronounced in patients with advanced MM with metastasis (Stage III, IV) than in patients with MM without metastasis (Stage I, II).These results suggest that comprehensive identification of autoantibodies in MM patients may be able to predict metastasis and progression.
Results
◎ The anti-topoisomerase I antibodies had been the most useful autoantibody for predicting interstitial lung disease (predicting interstitial lung disease in more than 70% of cases).
◎ The sum of autoantibody titers (SAL-P-CRP), which showed a significant positive correlation with CRP, has been shown to be a new biomarker that reflects the severity of scleroderma more usefully than anti-topoisomerase I antibodies.
◎ Comprehensive screening of autoantibodies across the proteome may reveal disease-specific immunological signatures and pave the way for the development of new diagnostic tools.
Paper information
Kazuki M Matsuda, et al. Autoantibody Landscape Revealed by Wet Protein Array: Sum of Autoantibody Levels Reflects Disease Status
Front Immunol. 2022 May 4:13:893086. doi: 10.3389/fimmu.2022.893086.

Comprehensive profiling of autoantibodies in systemic autoimmune diseases
Research Methodology
Protein synthesis
Sixty-five antigen proteins were selected to detect 43 autoantibodies associated with systemic sclerosis (SSc) and polymyositis (PM/DM) among systemic autoimmune diseases.

Fabrication of custom arrays
A custom protein array was created containing the 65 selected antigen proteins.The sera used were from 357 Japanese systemic sclerosis (SSc) patients, 172 Japanese polymyositis/dermatomyositis (PM/DM) patients, and 93 Japanese healthy subjects.

Autoantibody detection and analysis
Sera from SSc patients, PM/DM patients, and healthy individuals were analyzed using a custom array, and the most common antibodies detected in SSc patients were anti-CENP-B antibodies (32.2%), anti-topoisomerase I antibodies (26.9%), and anti-RNA polymerase III antibodies (11.2%).
In PM/DM patients, the most commonly detected antibodies were anti-TIF1-γ (24.4%), anti-TIF1-α (18.0%), and anti-PL-7 (6.4%).
In addition, anti-SS-A/Ro52 antibodies, which are associated with both diseases, were detected in 25.2% of SSc patients and 36.0% of PM/DM patients, whereas most autoantibodies were not detected in healthy individuals.

Auxiliary Verification
Autoantibody levels, such as anti-topoisomerase I antibody, anti-CENP-B antibody, and anti-RNA polymerase III antibody, were measured using commercially available ELISA kits.
Anti-AMA M2 antibodies were measured using a commercially available FEIA kit.
Antibodies were detected by immunoprecipitation.
The fluorescence pattern of autoantibodies was observed by cell staining using indirect antibody fluorescent method.
Correlation analysis was performed using the Spearman correlation test.
Results
◎ The custom array was able to detect 43 types of autoantibodies at once.This study demonstrates the utility of custom arrays and lays the foundation for comprehensive autoantibody profiling to improve diagnostic accuracy, enable earlier diagnosis, individualize treatment, and predict prognosis, and provide optimal healthcare tailored to patients.
Furthermore, important data was obtained that will contribute to a deeper understanding of the disease and the development of new treatments.
Paper information
Ai Kuzumi, et al. Comprehensive autoantibody profiling in systemic autoimmunity by a highly-sensitive multiplex protein array
Front Immunol. 2023 Aug 28:14:1255540. doi: 10.3389/fimmu.2023.1255540.

Identification and characterization of the antigen of TRA98 mouse monoclonal antibody that specifically binds to germ cells using a wet human comprehensive protein array
Research Methodology
Protein array development
We developed a comprehensive wet protein array (CWPA) containing 19,446 proteins synthesized using human cDNA as a template.CWPA has the world’s largest protein capacity and ability to prevent drying from production to assay.
We also achieved high uniformity in protein loading (the difference between the highest and lowest loading proteins was within an average of 47.3-fold).

Identification of mAb TRA98 antigen using comprehensive protein arrays
The mouse monoclonal antibody TRA98 (mAb TRA98) has the characteristic of specifically recognizing germ cell nuclei and has been widely used in germ cell research, but its antigen has remained unknown for over 20 years.
In this study, these authors used CWPA to identify the human antigen recognized by mAb TRA98, and found that mAb TRA98 specifically bound to nuclear factor-κB activating protein (NKAP).

Phenotype observation of mice lacking the Nkap gene function
To explore the role of NKAP protein in germ cells, these authors generated mice in which the Nkap gene was knocked out in the testis (Cre/loxP conditional deficient mice).
As a result, unlike wild-type mice, the testes were immature and lacked germ cells, exhibiting the pathology of Sertoli cell-retaining syndrome.

NKAP is SUMOylated in the testis
In the testes of transgenic mice carrying FLAG-tagged NKAP, the protein was detected as a 98 kDa protein by Western blotting using anti-FLAG antibody.
Since the molecular weight of the FLAG-tagged NKAP protein is 47 kDa, it was predicted that the protein would be modified in the testis, and this modification was suggested to be SUMOylation.
Results
◎By using CWPA, which has a high uniformity of protein loading, which was revealed that the antigen recognized by TRA98 in humans is NKAP.
◎ The testicular NKAP was SUMOylated and had a molecular weight of 98 kDa.
◎ It has become clear that NKAP plays an important role in testicular maturation.
Paper information
Eriko Fukuda, et al. Identification and characterization of the antigen recognized by the germ cell mAb TRA98 using a human comprehensive wet protein array
Genes Cells. 2021 Mar;26(3):180-189. doi: 10.1111/gtc.12832.

Case 5: Discovery of a new autoantibody marker (anti-IFI16 antibody) in idiopathic interstitial pneumonia (IIP) and clinical characteristics in a multicenter cohort study
Research Methodology
Autoantibody screening in idiopathic interstitial pneumonia (IIP)
Autoantibody screening was performed using 35S-methionine-labeled protein immunoprecipitation in 295 patients diagnosed with idiopathic interstitial pneumonia.
As a result, these authors found that a common tetrameric protein was immunoprecipitated in the sera of 6 out of 295 IIP patients.
To identify the antigen in this tetramer, HuPEX® comprehensive protein array analysis was used, and interferon gamma-inducible protein 16 (IFI16 protein) was identified.

Confirmation of protein synthesis and antigen-antibody reaction of interferon-gamma-inducible protein 16 (IFI16)
To confirm that autoantibodies against the tetrameric protein specifically recognize the IFI16 protein, these authors synthesized IFI16 protein by in vitro transcription-translation (IVTT) using a plasmid carrying the IFI16 coding sequence (CDS) and performed immunoprecipitation. As a result, IFI16 was confirmed to be precipitated in all six patient sera that reacted to the tetrameric protein, confirming that IFI16 is the target antigen.

Clinical features associated with anti-IFI16 antibodies
Among IIP patients, those in whom anti-IFI16 antibodies are detected have a lower opportunity to receive immunosuppressive therapy and may have a poorer prognosis.Further studies are needed to improve patient stratification and management.
Results
◎These authors discovered a novel autoantibody marker in IIP, anti-IFI16 antibody, and used HuPEX® comprehensive protein arrays to identify the antigen of this autoantibody.
◎The research clarifying the clinical significance of anti-IFI16 antibodies may lead to prediction of therapeutic response, the possibility of personalized treatment, and a deeper understanding of the immunological background of the disease, which may contribute to the development of more appropriate treatments.
Paper information
Tsuneo Sasai, Ran Nakashima, et al. Anti-interferon gamma-inducible protein 16 antibodies: Identification of anovel autoantigen in idiopathic interstitial pneumonia and its clinicalcharacteristics based on a multicenter cohort study
Clin Immunol. 2024 Nov:268:110372. doi: 10.1016/j.clim.2024.110372.

Association between epitope suppression (ES) of anti-RNA polymerase III antibodies and clinical symptoms of systemic sclerosis
Research Methodology
Protein expression
To analyze autoantibodies in the sera of systemic sclerosis (SSc) patients, we synthesized partial proteins of subunits of the RNA polymerase III (RNAP III) complex and the RNAPIII major antigen RPC1.
Seventeen full-length proteins of RNAP III complex subunits and five partial proteins of RPC1 were synthesized as FLAG-GST fusion proteins using a wheat germ cell-free protein synthesis system.

Preparation of antigen-binding plates
Using the synthesized proteins, we created antigen-binding plates using our proprietary technology.Each well of the antigen-binding plate (96-well plate) is coated with glutathione (GSH) to allow binding of FLAG-GST fusion proteins.

Detection and quantification of autoantibodies
Serum samples from 75 SSc patients, including 34 anti-RNA polymerase III antibody (ARA) positive SSc patients, were used to measure autoantibodies against 17 subunits of the RNAP III complex and five partial proteins of the major antigen RPC1.
As a result, autoantibodies against various subunits of the RNAP III complex were detected in ARA-positive SSc patients, and showed different patterns in each patient.
Therefore, we quantified and compared the intermolecular epitope spreading for subunits of the RNAP III complex and the intramolecular epitope spreading for the major epitope RPC1.

Statistical analysis and correlation with clinical features
We also analyzed the epitope distribution of the quantified RNAP III complex subunits and the major epitope RPC1 and its association with clinical findings.Interestingly, the greater the spread of the epitope across the subunits, the more significant the correlation with skin sclerosis (mRSS) and SP-D (a marker for interstitial pneumonia) in the Spearman correlation coefficient.
In addition, we found that the greater the epitope spread to the major epitope RPC1, the higher the risk of developing renal crisis.
When the spread of the epitope was evaluated over time in the same patient, which was found to be linked to the degree of skin sclerosis, suggesting that it may be useful as a marker of disease activity in SSc.
Results
◎We showed that antibodies targeting subunits of the RNAP III complex correlated with disease severity and organ damage (skin and lung) in SSc patients, suggesting that these antibodies may be useful biomarkers for early diagnosis and prognostic evaluation.
※Epitope spreading (ES) refers to the phenomenon in which, in autoimmune diseases, autoantibodies against a specific antigen are initially formed, and then autoantibodies against different antigens are gradually formed.
Paper information
Diversity and Epitope Spreading of Anti-RNA Polymerase III Antibodies in Systemic Sclerosis: A Potential Biomarker for Skin and Lung Involvement Hirohito Kotani et al., Arthritis & Rheumatology, 2024. doi: 10.1002/art.42975
Frequently asked questions and inquiries
We have compiled a list of frequently asked questions from customers. If you have any questions that are not answered here, please feel free to contact us using our inquiry/quote form.
HuPEX® comprehensive protein array analysis service Related Papers
List of papers
Artificial intelligence and omics-based autoantibody profiling in dementia
Front. Immunol., 08 May 2025 Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
The enigma of anti-transcobalamin receptor autoantibodiesUnveiling the hidden syndrome: The enigma of anti-transcobalamin receptor autoantibodiesThe enigma of anti-transcobalamin receptor autoantibodies
Immunology Letters. Volume 275, October 2025, 107028
Proteome-Wide Analysis of Autoantibodies in Open-Angle Glaucoma in Japanese Population: A Pilot Study
Biomedicines . 2025 Mar 14;13(3):718.
A Case of Acquired Idiopathic Generalized Anhidrosis with Anti-mucin 7 Seropositivity
Acta Dermato-Venereologica, 105, adv42883. Feb 5, 2025
Diversity and Epitope Spreading of Anti-RNA Polymerase III Antibodies in Systemic Sclerosis: A Potential Biomarker for Skin and Lung Involvement
Arthritis Rheumatol. 2024 Sep 1. doi: 10.1002/art.42975
HuPEX® comprehensive protein array Related Papers
List of papers
testComprehensive autoantibody profiling in systemic autoimmunity by a highly-sensitive multiplex protein arraytest
Front Immunol. 2023 Aug 28:14:1255540. doi: 10.3389/fimmu.2023.1255540. eCollection 2023.


